© 2024 Tolmar, Inc. All rights reserved. Tolmar, ELIGARD and their associated logos are trademarks of the Tolmar Group. Third-party
trademarks and product names belong to their respective owners. TPI.2023.eng.4010.v3 06/25
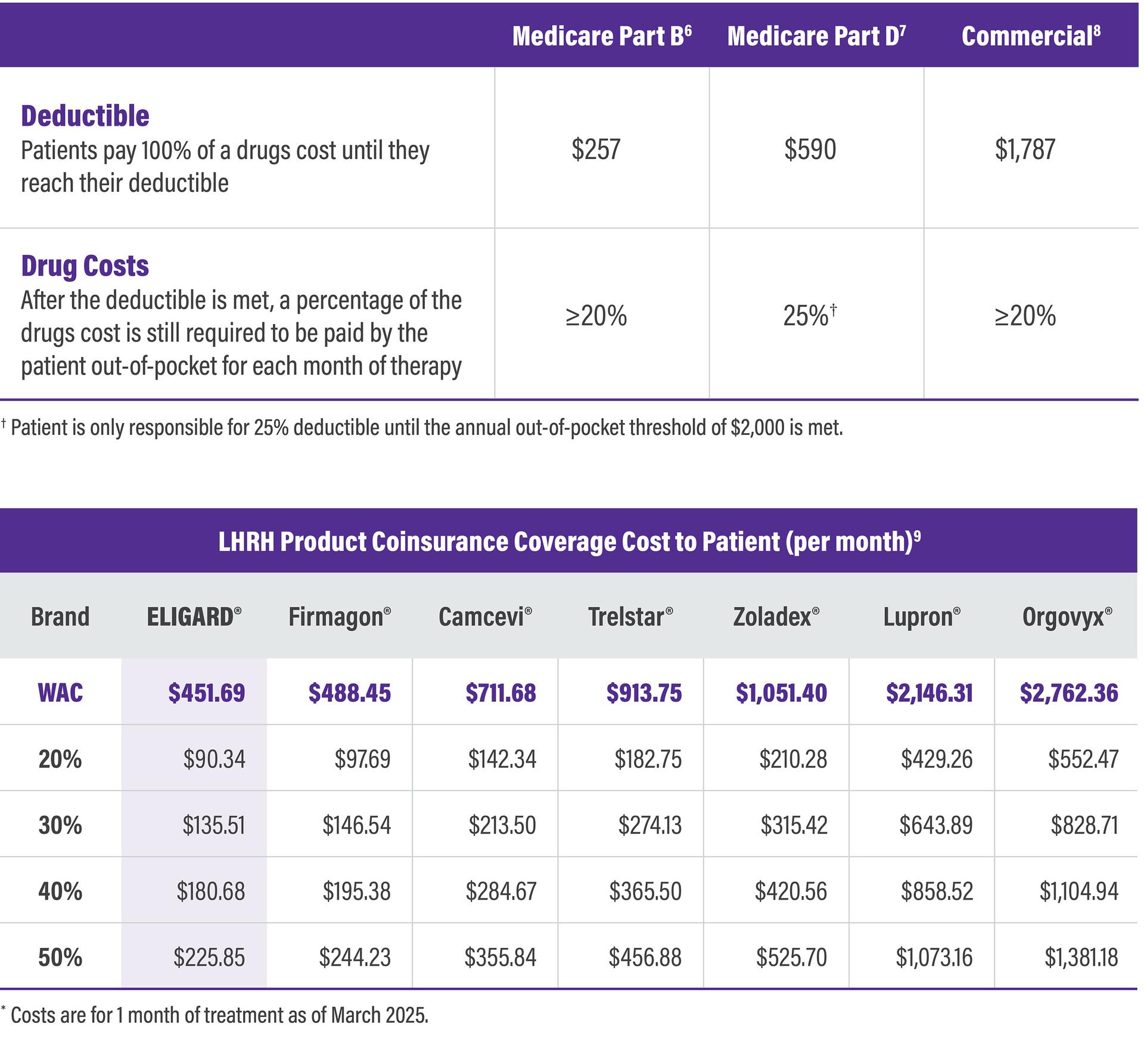
Higher WAC products can cause a higher cost burden to patients
The impact of escalating costs on individual patients with prostate cancer
WAC = Wholesale acquisition cost

Cancer is one of the most expensive medical conditions to treat in the US and often leads to financial toxicity that can impact care.
- Patients requiring oral cancer drugs may be particularly vulnerable to high out-of-pocket spending, as these medications are often associated with high co-payments or coinsurance, particularly in plans with tiered prescription formularies.1-3
- Up to 64% of cancer patients reported increased stress from worrying about whether they can pay their medical bills and out-of-pocket costs, also known as financial toxicity.4
- One study even showed almost 40% of cancer survivors had to make other kinds of financial sacrifices because of their cancer, its treatment, or the lasting effects of treatment.5

Patient Cost Burden by Coverage Type*
If treatment cost is high, perhaps see if a similar, more responsibly priced product is available with similar efficacy and discuss with the provider if that alternative product is available and right for that patient.
Do you consider the financial side effect of the product you select when starting a patient on ADT?
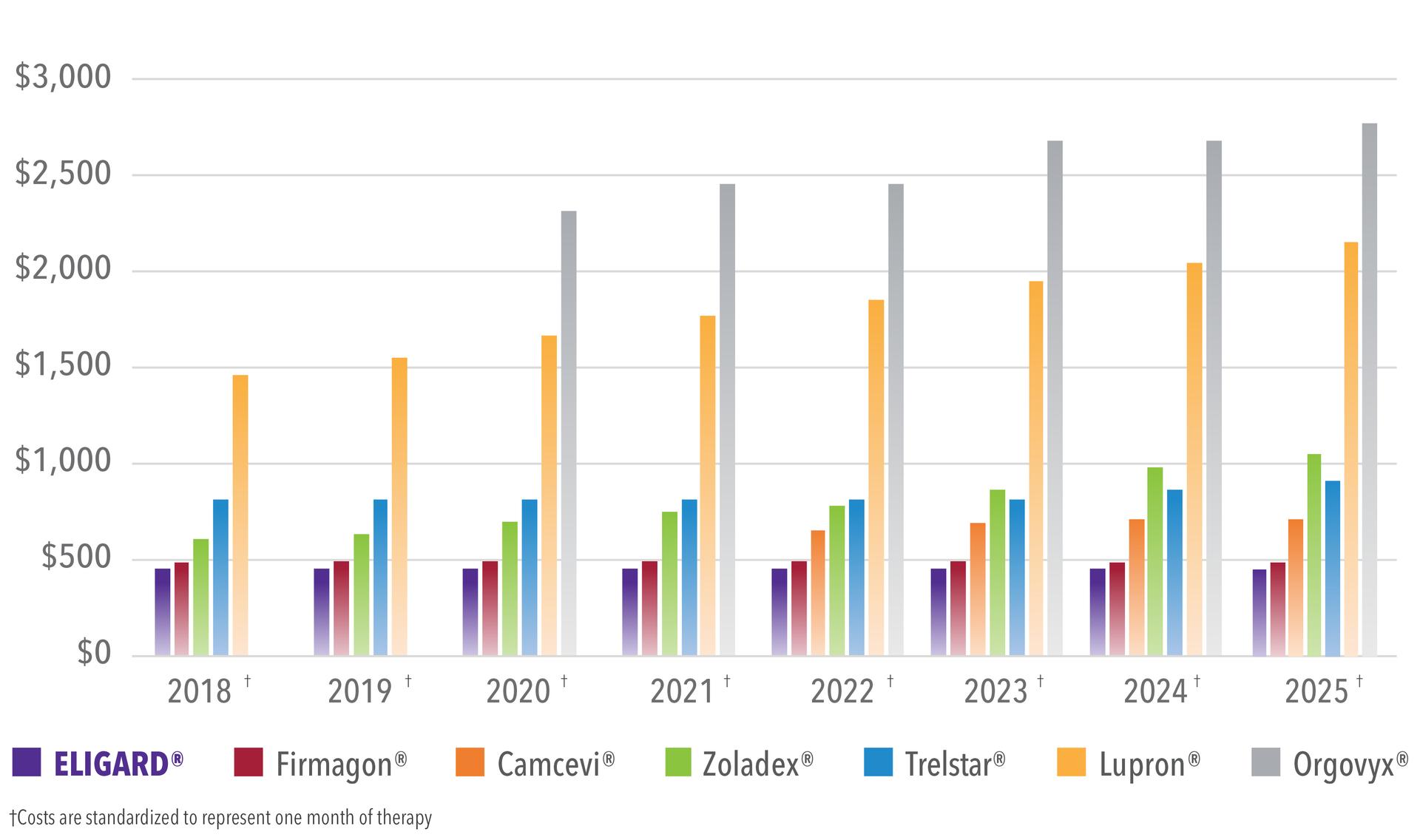
ELIGARD has consistently had one of the lowest WACs in the LHRH market, ensuring that cost burden to the patient is kept as low as possible.

Tolmar is meeting the needs of patients and providers
Tolmar is committed to keeping ELIGARD responsibly priced to ensure access across healthcare systems, providers, and most importantly – patients
Patient Access Support
Practice Support
Clinical Support
Low OOP Expense
To learn more about ELIGARD’s proven efficacy and flexible dosing visit EligardHCP.com
IMPORTANT SAFETY INFORMATION
ELIGARD®, (leuprolide acetate) for injectable suspension, is a gonadotropin releasing hormone (GnRH) agonist indicated for the treatment of advanced prostate cancer.
ELIGARD is contraindicated in patients with hypersensitivity to GnRH, GnRH agonists, or any of the components of ELIGARD. Anaphylactic reactions to synthetic GnRH or GnRH agonists have been reported in the literature. Transient increase in serum levels of testosterone during treatment may result in worsening of symptoms or onset of new signs and symptoms during the first few weeks of treatment, including bone pain, neuropathy, hematuria, bladder outlet obstruction, ureteral obstruction, or spinal cord compression. Monitor patients at risk closely and manage as appropriate.
Hyperglycemia and an increased risk of developing diabetes have been reported in men receiving GnRH agonists. Monitor blood glucose level and manage according to current clinical practice. Increased risk of myocardial infarction, sudden cardiac death and stroke has also been reported with use of GnRH agonists in men. Monitor for cardiovascular disease and manage according to current clinical practice. Androgen deprivation therapy may prolong the QT/QTc interval. Consider risks and benefits. May cause fetal harm. Convulsions have been observed in patients taking leuprolide acetate with or without a history of predisposing factors. Manage convulsions according to current clinical practice.
ELIGARD may impair fertility in males of reproductive potential.
The most common injection site adverse events are transient burning and stinging, pain, bruising, and erythema. The most common systemic adverse events include mild to severe hot flashes/sweats, malaise and fatigue, weakness, myalgia, dizziness, clamminess, testicular atrophy, and gynecomastia. As with other GnRH agonists, other adverse reactions,
including decreased bone density and rare cases of pituitary apoplexy have been reported. Please visit EligardHCP.com for full Prescribing and Safety Information
References:
1. Khan HM, et.al. Financial Toxicity in Cancer Care: Implications for Clinical Care and Potential Practice Solutions. J Clin Uro. 2023;41(16) https://doi.org/10.1200/JCO.22.0179
2. Kaiser Family Foundation. 2020 Employer Health Benefits Survey. KFF.org. Published Oct 8, 2020. Accessed May 7, 2025 https:https://www.kff.org/report-section/ehbs-2020-section-9-prescription-drug-benefits/
3. Werble, C. Formularies. Health Affairs Policy Brief. healthaffairs.org. Published Sep 14, 2017. Accessed May 7, 2025 https://www.healthaffairs.org/content/briefs/formularies
4. NIH National Cancer Institute. Financial Toxicity and Cancer Treatment (PDQ)-Health Professional Version. Cancer.gov. Updated May 29, 2024. Accessed Apr 30, 2025 https://www.cancer.gov/about-cancer/managing-care/track-care-costs/financial-toxicity-hp-pdq#_215_toc
5. Banegas MP, Guy GP, de Moor JS, et al. For Working-Age Cancer Survivors, Medical Debt And Bankruptcy Create Financial Hardships. Health Aff (Millwood) 2016;35 (1): 54-61 https://doi.org/10.1377/hlthaff.2015.0830
6. US Railroad Retirement Board. 2025 Medicare Part B Premiums and Deductibles Will Increase. RRB News. rrb.gov. Published Nov 2024. Accessed May 7, 2025 https://www.rrb.gov/sites/default/files/2024-11/NR2410.pdf.
7. Centers for Medicare & Medicaid Services. Final CY 2025 Part D Redesign Program Instructions Fact Sheet. cms.gov. Published Apr 1, 2024. Accessed May 7, 2025 https://www.cms.gov/newsroom/fact-sheets/final-cy-2025-part-d-redesign-program-instructions-fact-sheet/
8. Kaiser Family Foundation. 2024 Employer Health Benefits Survey. kff.org. Published Oct 9, 2024. Accessed May 7, 2025 https://www.kff.org/report-section/ehbs-2024-summary-of-findings/
9. Kaiser Family Foundation. A Current Snapshot of the Medicare Part D Prescription Drug Benefit. kff.org. Published Oct 9, 2024. Accessed May 7, 2025 https://www.kff.org/medicare/issue-brief/a-current-snapshot-of-the-medicare-part-d-prescription-drug-benefit/
10. Data on file, Tolmar, Inc.; 2025


